Research focus
Our research is mainly focused on:
- Pancreatic cancer biology and resistance mechanisms.
- Tumor modeling based on patient-derived organoids and cancer associated fibroblasts.
Despite major advances in oncological as well as surgical therapy, the prognosis of pancreatic cancer remains poor due to late diagnosis and high resistance to current chemotherapies. Our laboratory focuses on translational research of pancreatic cancer as the basis for the development of more effective, individualized therapy concepts. Using next-generation sequencing technologies, we integrate detailed clinical and molecular data from patient samples in order to identify molecular alterations associated with tumor progression, resistance to therapy and survival. Subsequently, we aim to investigate if these alterations can be used as prognostic and therapy-stratification biomarkers as well as new therapeutic targets.
We use primary 3D organoid cultures generated from fresh tumor samples to investigate in vitro pancreatic cancer biology and resistance mechanisms. Additionally, we aim to validate organoids models as patient-specific predictive tools of chemosensitivity and to functionally test new drugs and therapeutic targets. Furthermore, we are developing mouse models to validate in vitro targets in vivo.
Our ultimate goal is the development and validation of new individualized therapy approaches for pancreatic cancer.
Current Projects
- 3D co-culture models of patient-matched pancreatic tumor organoids and cancer associated fibroblasts to investigate the impact of stroma on chemoresistance in pancreatic cancer.
- Immunofunctionalization of in vitro tumor models.
- Modeling structural genomic aberrations and their role in pancreatic cancer biology and treatment resistance.
- Investigation of resistance mechanisms triggered by chemotherapeutic agents in pancreatic tumor organoids.
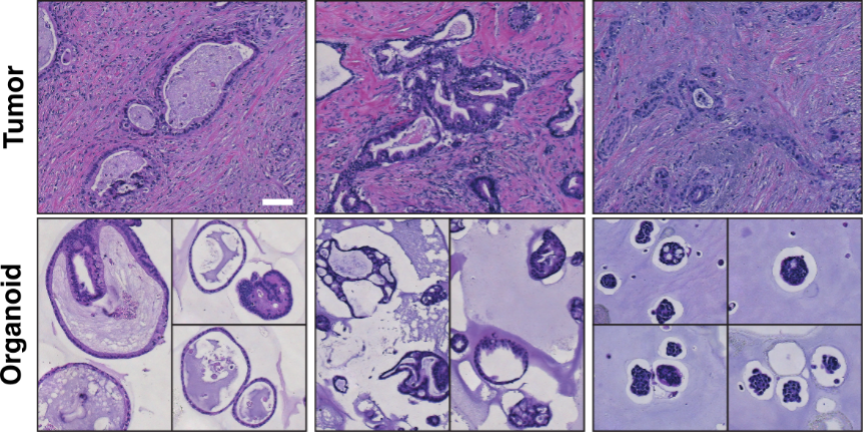
PDAC organoid growth patterns show resemblance to the epithelial architecture of the original tumor. Hematoxylin and eosin staining, scale bar 100 µm.
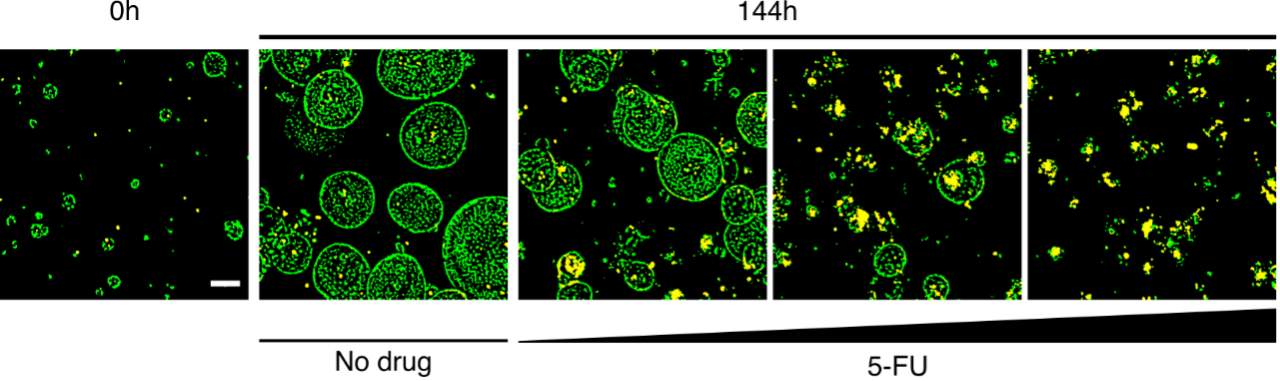
A live confocal image-based drug assay allows to resolve drug‐induced cell death and growth inhibition in organoids. Images show organoids before (0h) and after (144h) treatment with increasing concentrations of the chemotherapeutic drug 5-FU. Living cells appear in green (Hoechst) while death cells appear in yellow (Hoechst + propidium iodide). Scale bar: 100 µm. Images by Julia Jabs, German Cancer Research Center.
Team

Prof. Dr. Oliver STROBEL, MD, MBA
Principal Investigator
Professor for Visceral Surgery
Head of the Department of General Surgery
Head of the Division of Visceral Surgery
oliver.strobel@meduniwien.ac.at

Solange LE BLANC SOTO, PhD
Co-Investigator

Carl LEONHARDT, MD, MBA
Co-Investigator

Carina BINDER, MD
Predoctoral Fellow
Publications
Persister cell phenotypes contribute to poor patient outcomes after neoadjuvant chemotherapy in PDAC. Zhou X, An J, Kurilov R, Brors B, Hu K, Peccerella T, Roessler S, Pfütze K, Schulz A, Wolf S, Hohmann N, Theile D, Sauter M, Burhenne J, Ei S, Heger U, Strobel O, Barry ST, Springfeld C, Tjaden C, Bergmann F, Büchler M, Hackert T, Fortunato F, Neoptolemos JP, Bailey P. Nat Cancer. 2023 Sep;4(9):1362-1381. doi: 10.1038/s43018-023-00628-6
Shifting the focus of zebrafish toward a model of the tumor microenvironment. Weiss J, Lumaquin-Yin D, Montal E, Suresh S, Leonhardt CS, White RM. Elife. 2022 Dec 20:11:e69703. doi: 10.7554/eLife.69703.
Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system. Schuth S, Le Blanc S, Krieger TG, Jabs J, Schenk M, Giese NA, Büchler MW, Eils R, Conrad C, Strobel O. J Exp Clin Cancer Res. 2022 Oct 22;41(1):312. doi:10.1186/s13046-022-02519-7
Single-cell analysis of patient-derived PDAC organoids reveals cell state heterogeneity and a conserved developmental hierarchy. Krieger TG, Le Blanc S, Jabs J, Ten FW, Ishaque N, Jechow K, Debnath O, Leonhardt CS, Giri A, Eils R, Strobel O, Conrad C. Nat Commun. 2021 Oct 5;12(1):5826. doi: 10.1038/s41467-021-26059-4
Preprint: Deep molecular characterization linked to drug response profiling of pancreatic ductal adenocarcinoma using patient-derived organoids. Le Blanc S, Ishaque N, Jabs J, Bauer T, Schuth S, Hu Q, Debnath O, Ten FW, Leonhardt CS, König A, Bieg M, Eckert C, Gaida MM, Volkmar M, Hübschmann D, Schenk M, Offringa R, Giese NA, Schlesner M, Büchler MW, Eils R, Conrad C, Strobel O. bioRxiv 2021.08.26.457743; doi: 10.1101/2021.08.26.457743
